hello my friends. doctor steemit is here again now 21000sp power more 2$ upvote makes the profitable post for you and you can get the information about the spectrophotometry waht is the method and machine of spectrophotmetry.
Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The basic principle is that each compound absorbs or transmits light over a certain range of wavelength. This measurement can also be used to measure the amount of a known chemical substance.
absorbance and the Beer-Lambert Law are reviewed in addition to the components of the spectrophotometer. These concepts provide a foundation for how to determine the concentration of a solute in solution that is capable of absorbing light in the ultraviolet and visible range.
Electromagnetic radiation is characterized by its frequency (n) or its wavelength (l).These two are related by the velocity of light (c),
n=c/l.
The Spectronic 20 spectrometer is widely used in teaching laboratories. The specific instructions will differ with other models, but the principles remain.
The instrument must have been warm for at least 15 min. prior to use. The power switch doubles as the zeroing control.
Use the wavelength knob to set the desired wavelength. Extreme wavelengths, in the ultraviolet or infrared ranges, require special filters, light sources, and/or sample holders (cuvettes).
major sequence of events in spectrophotometry is as follows:
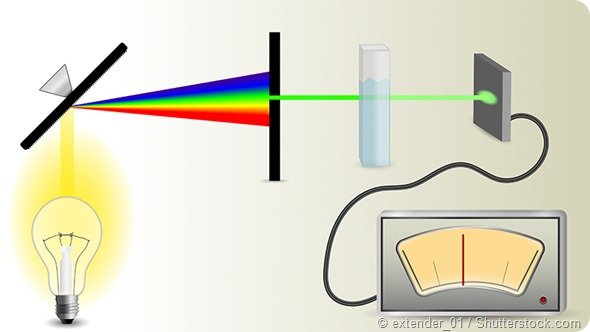
The light source shines through a monochromator.
An output wavelength is selected and beamed at the sample.
A fraction of the monochromatic light is transmitted through the sample and to the photo-detector.
What is the difference between UV/Vis vs. fluorescence measurements?
UV/Vis measurements occur in the ultraviolet (UV) and visible portion of the electromagnetic spectrum. UV/Vis measurements can directly measure the concentration of a molecule with a known extinction coefficient or indirectly measure a concentration utilizing a standard curve.
Spectrophotometric determination of protein concentration:
The concentration of a purified protein in solution is most conveniently and accurately measured using absorbance spectroscopy. The absorbance, A, is a linear function of the molar concentration, C, according to the Beer-Lambert law: A = epsilon x l x c, where e is the molar absorption coefficient and l is the cell path length. This unit provides protocols for calculation of epsilon for a folded or unfolded protein,
.gif)
So how does a spectrophotometer work? What's going on inside the box?
partes:
A sample solution is placed inside the spectrophotometer.
A light source shines light toward the sample.
A device called a monochromator splits the light into each color, or rather, individual wavelengths (just like a raindrop makes a rainbow). An adjustable slit allows only one specific wavelength of light through to the sample solution.
The wavelength of light hits the sample, which is held in a little container called a cuvette. We need to be careful when handling cuvettes; even a slight fingerprint can interfere with the results.


 \
\


nice post about the chemistry and useful information. we can know how is the concentration of the protein in one sample with use this nice machine thanks for sharing nice information
nice comment again my friend thanksss for your gift and thankss for useful comment
Sources:
http://onlinelibrary.wiley.com/doi/10.1002/0471140864.ps0301s33/abstract;jsessionid=FF451EC50FCEDE1A8C7566B68E0E33CF.f04t01
https://brainly.in/question/1162605
Not indicating that the content you copy/paste is not your original work could be seen as plagiarism.
Some tips to share content and add value:
Repeated plagiarized posts are considered spam. Spam is discouraged by the community, and may result in action from the cheetah bot.
Creative Commons: If you are posting content under a Creative Commons license, please attribute and link according to the specific license. If you are posting content under CC0 or Public Domain please consider noting that at the end of your post.
If you are actually the original author, please do reply to let us know!
Thank You!
More Info: Abuse Guide - 2017.
Doctor Steemit you are a cool dude. Enjoyed your post I got lost a little so read it again.
Could you use something like that to do non-invasive blood tests?
thanks for your comment i will try to make it
Really wow I will be the first to buy it, I am sick of putting holes in my fingers every day to test my blood glucose level
you explained it well, nice job
Thankyou
thanks for read my article