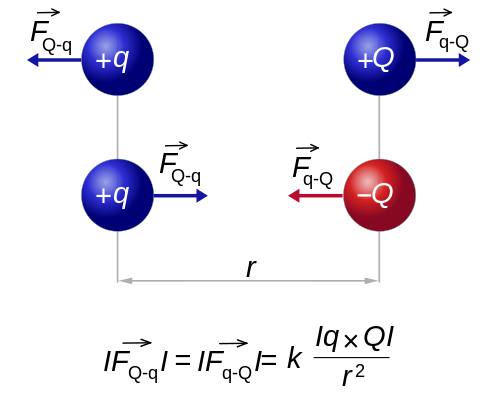
The magnitude of each of the electric forces with which two point charges at rest interact is directly proportional to the product of the magnitude of both charges and inversely proportional to the square of the distance that separates them. Charles Augustin de Coulomb (1736-1806) quantitatively measured electrical attraction and repulsion and deduced the law that governs them. The Law of Coulomb, which establishes what the force is between two point electric charges, constitutes the starting point of Electrostatics as a quantitative science.
It was discovered by Priestley in 1766, and rediscovered by Cavendish a few years later, but it was Coulomb in 1785 who subjected it to direct experimental trials.
We understand by punctual charge an electric charge located in a geometric point of space. Obviously, a point load does not exist, it is an idealization, but it is a good approximation when we are studying the interaction between electrically charged bodies whose dimensions are very small compared to the distance between them.
The Law of Coulomb says that
"the electrostatic force between two point charges is proportional to the product of the charges and inversely proportional to the square of the distance that separates them, and has the direction of the line that joins them. the charges are of the same sign, and of attraction if they are of the opposite sign "
Development of the law
Coulomb developed the torsion balance with which he determined the properties of the electrostatic force. This instrument consists of a bar that hangs from a fiber capable of twisting. If the bar rotates, the fiber tends to return it to its original position, so that knowing the torsional force that the fiber exerts on the bar, the force exerted on a point of the bar can be determined. The law of Coulomb also known as the law of charges has to do with the electrical charges of a material, that is, it depends on their charges are negative or positive. In the bar of the balance, Coulomb placed a small charged sphere and then, at different distances, positioned another sphere also charged. Then he measured the force between them by observing the angle that the bar rotated.
These measurements allowed to determine that:
The force of interaction between two charges q1 and q2 doubles its magnitude if one of the charges doubles its value, triples it if one of the charges increases its value by a factor of three, and so on. He concluded that the value of the force was proportional to the product of the charges: Fα q1 and Fα q2 consequently Fα q1q2 If the distance between the charges is R.png by duplicating it, the interaction force decreases by a factor of 4 (2²); when tripled, it decreases by a factor of 9 (3²) and by quadrupling the force between loads decreases by a factor of 16 (4²). Consequently, the force of interaction between two point charges is inversely proportional to the square of the distance:
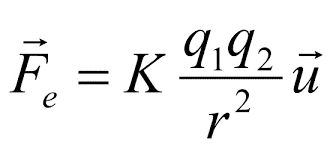
It is then defined that the force of attraction with which objects interact depends on their electric charge and whether it is positive or negative. The sign of that charge develops in the envelope of its nucleus, that is, every electrical phenomenon is composed of an atom, which has a nucleus formed by protons (positive charge) and neutrons (without charge), and surrounded by electrons (charge) negative). The interaction of its own composition will then define the force of attraction to be in the presence of another objective that has electric charge.
If both charges have the same sign, that is, if both are positive or both negative, the lines of force repel each other. On the contrary, if the two charges have opposite signs, the lines of force are attracted.
An example of the interaction between positive and negative charges, can be seen with magnets that work with magnetism and not with electric charges, they have this same principle, where two magnets of equal charges are repelled, while those with charges opposite join.
Finally, it must be taken into consideration that this law can only be applied with objects that have an electric charge, which are small in relation to the distance that separates them and that are static (without movement), that's why the Law of Coulomb is also known as electrostatic.
