The aggregation states:
According to experimental evidences, the different states of aggregation have common properties, although they are different substances as well as identical behaviors.
Solids
In its own way, and some regular.
They are practically not compressed, so their volume is constant.
Its density is quite close to that of liquids.
They do not flow.
Liquids
Adopt the shape of the container that contains them.
They are compressed with difficulty, so their volume is practically constant.
They are more dense than gases.
They can flow.
Gases
*They do not have their own form.
They compress easily and expand by filling the volume of the container that contains them.
Their densities are very low compared to that of liquids and solids.
They can flow.
Exercise forces on all parts of the container that contains them.
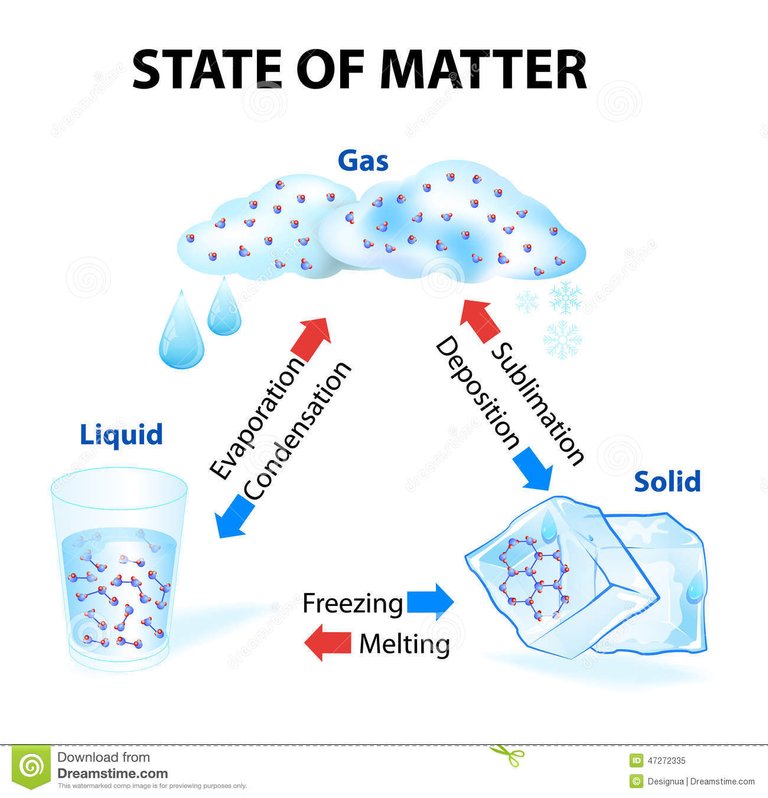
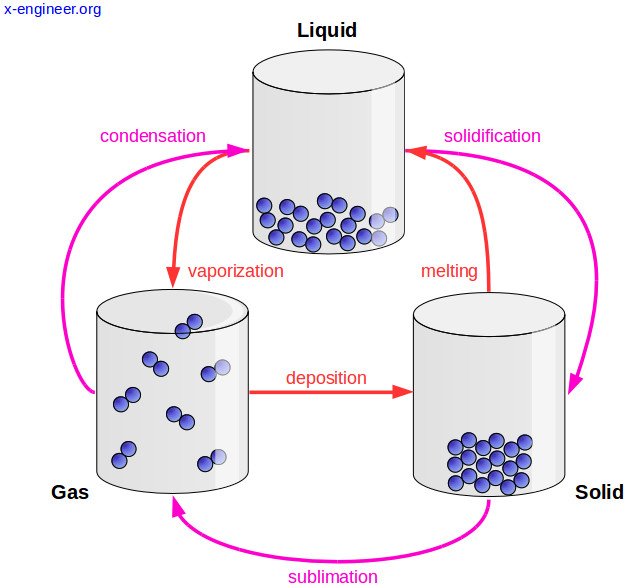
Changes of state
At room temperature, each substance is in a certain state; water is liquid, oxygen is gaseous, iron is solid. But at other temperatures, substances change state: water can be solid; mercury, gaseous, and oxygen, liquid for example. Thus, matter changes state according to the temperature at which it is. Below are the names of the different state changes.
Source
Melting
All solids melt at a characteristic temperature called temperature or melting point, which is different for each solid. While the melting lasts, the temperature remains constant.
Evaporation
Evaporation is the passage of liquid to gas at any temperature below the boiling temperature; It is a phenomenon that occurs on the surface of any liquid, some do it slowly, like oil, and others very quickly, like alcohol. The higher the temperature, the higher the evaporation rate.
Condensation
The passage from the gaseous state to the liquid state is called condensation. The temperature at which the passage of water vapor to liquid (condensed) water occurs is called the dew point. Droplets of water form on the walls of the funnel. This phenomenon is called condensation and it is very easy to observe it around us; in the fogging of the glass of the cars, in a mirror, in the drops of dew on the plants, on the surface of the glasses with ice ...
Sublimation
The direct passage from solid to gas, without passing through liquid is called direct sublimation. The kinetic theory explains this change of state because the particles that are on the surface of the solid constantly move, have enough energy to escape and go to the gaseous state, without going through the molten solid state. Likewise, when some gases are cooled, they can be converted directly into solids, without passing through liquid. It's the reverse sublimation.
Kinetic theory
The properties of the different states of aggregation are well explained by the kinetic-corpuscular theory of matter (kinetic theory).
A model of matter: the kinetic theory
The behavior of matter is currently explained with the kinetic theory, based on the following assumptions:
All matter is composed of very small particles in continuous movement.
An increase in temperature causes an increase in the energy of the particles.
The size of the particles is very small compared to the distance between them.
The particles move in all directions much more quickly when the temperature is higher. In the case of a gas, they continuously collide with the walls of the container that contains them. The result of these shocks is the gaseous pressure.
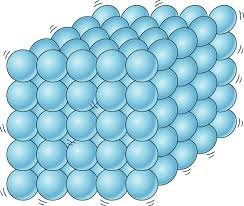
The solids are formed by particles joined by quite strong forces. The particles are in fixed positions and have only vibration movements around those fixed positions.
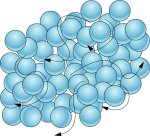
Liquids are formed by particles joined by weaker forces than in solids. The particles can move from one point to another, although they remain attached. The difference with solids is that there are some holes and this creates free spaces where particles can jump. That's why liquids flow.
The gases are formed by particles more separated from each other, since the forces are negligible. Therefore, they are not very dense and compress easily. The particles have a totally disordered movement. As a result of this movement they collide on the walls of the container exerting force on them.
The kinetic theory explains the changes of state.
The boiling
When heating the liquid, the particles will be carried out one on top of the other with increasing speed. And there will come a time when the attractive forces that held them together will be overcome, and the set of particles will begin to separate. The substance has reached its boiling temperature. The heat that is provided from that moment is used to break the joints of the particles, so the temperature does not change. Once all the joints have been broken, the particles, already separated, increase their speed again. The boiling point is a characteristic property of matter, knowing the boiling point we can recognize what material it is.
Evaporation
The average velocity of the particles depends on the temperature of the liquid. On the surface of the liquid, the temperature is higher; therefore, there are more particles that have enough velocity to escape and go to the vapor state. The wider the surface of the container, the more particles will have the possibility of being on top and escaping.
References
https://study.com/academy/lesson/phase-change-evaporation-condensation-freezing-melting.html
https://chemstuff.co.uk/academic-work/year-7/particle-model-of-solids-liquids-and-gases/
https://www.le.ac.uk/se/centres/sci/selfstudy/particle02.html



.jpeg)