These series are going to be "tiny" posts about some topics I need to study for my exams.
They are going to be an "experiment" so I can prepare the Final Exam Questions & write a post at the same time. By doing these, I can keep you updated with science content while I also study as I explain the topic for a post. These are not going to be a substitution of my full length researches but rather a temporary work-around to keep posting while I am more busy.
Without any other distraction, let's dive into it!
Sweeteners
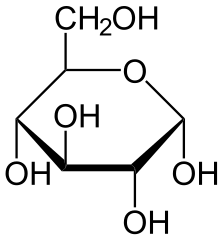
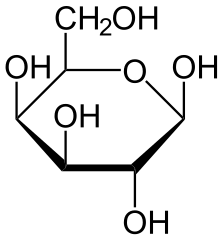
The compounds that cause the sensation of sweetness in our taste are sugars like Glucose or Galactose, but this same response can be triggered by other compounds derived from plants that can actually make the sweet flavour stronger while adding zero to the caloric intake!
Sweeteners are just substitutions of these sugars that can cause the same reaction in our taste buds. For that, these compounds have to be also soluble in water because otherwise they will not be able to interact with our taste buds.
Glucose (up) by NEUROtiker(Public Domain) & Galactose (down) by NEUROtiker (Public Domain)
Interesting Fact: If you read the food composition, you can see names with numbers like E9XX. These indicate there is a sweetener present!
The list goes below with the following characteristics:
Polyols (E967)
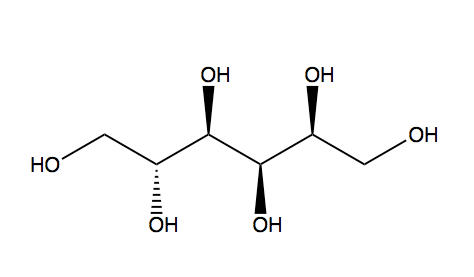
These are sugar alcohols or compounds derived from sugar which have multiple Hydroxyl groups (-OH).
They are in general less sweet and caloric than glucose.
Sorbitol, Mannitol an Xylitol are examples of these type (even though Xylitol is 10 times sweeter).
Saccharin (E954)
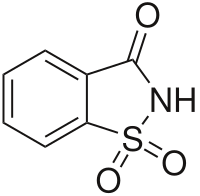
This one is surprisingly 300 times sweeter and it could be used in most food. However, it leaves a metallic taste afterwards. Unfortunately, it can be carcinogenic so it is not used that much any longer.
Acesulfame K (E950)
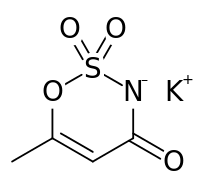
This sweetener is 200 times more sweet than normal sugar, doesn't leave an after-taste like Saccharin and most imporantly it odesn't get metabolised (so it doesn't affect your caloric intake!).
Aspartame (E951)
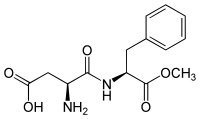
This sweetener consists of 2 peptides: aspartic acid methylester of phenylalanine. It is one of the most used as it doesn't have any side effect. It has a sweetness 180 times higher than normal sugar.
However, patients with phenylketonuria have to bear in mind if their food have this sweetener.
Stevia (E960)

Stevia Plant - by Ethel Aardvark (CC BY 3.0)
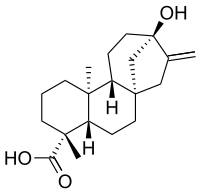
If you have ever seen Breaking Bad you can remember this name. It is a compound 250 times sweeter than sugar, and it is derived form the plant shown above.
The compound is a glycoside, which is a sugar bounded to another functional group (the ones responsible for the reactions). It's molecule is shown in the picture on the right.
Thaumatin (E957)
It is form by different polypeptides (proteins) and it is derived from an african fruit called Thaumatococcus Daniellii from West Africa. It's perception lasts a long time but it leaves a liquour after-taste. Surprisingly it is 2000 times more sweet than normal sugar!
Sucralose (E950)
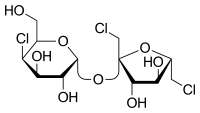
This last sweetener is a chlorinated sugar which is 600 times more sweet than normal sugar. It is formed by a sucrose molecule with 3 -OH substituted by Cl.
It is 600 times sweetener than normal sugar.
Closing
I hope you liked the post and learned something new and different. I will try to keep updated with new content as I continue studying for my Finals, but if it doesn't work for me, I would have to stop alltogether. (Wish me luck!)
Some feedback would be appreciated in the format/quality as well.
However I would like to continue doing my big research posts as I have some ideas that would be great to share when I have time to prepare them!
Signing off!
Anyway, this is @deholt,
References
- Polyol - Wikipedia
- Saccharin - Wikipedia
- Carcinogenicity of saccharin. - by M D Reuber
- Acesulfame K - Wikipedia
- Aspartame - Wikipedia
- Stevia (plant) - Wikipedia
- Thaumatin - Wikipedia
- Sucralose - Wikipedia

Very smart way to go about this. Kill two birds with one stone. I wish steemit was around when I was studying accounting would had some interesting business and accounting posts to make. Best of luck on your exams! I don’t miss those days at all haha.