Introduction
In 1926, German physicist Werner Heisenberg (Not Walter White) at the age of 24 published a paper which made a controversial argument in quantum mechanics. In simple words, he claimed that one can never measure the position and momentum of a quantum particle at the same time.
This was a shocking statement from Heisenberg at the time, but what did he really mean by that? And why can't you measure the properties simultaneously? Is there something we don't know? It turns out there is.
The uncertainty principle states that it is impossible to know the position or the momentum of a particle. We can either know the position or the momentum.
But the term uncertainty is misleading here, because it leads to people thinking it's impossible to know both properties because we aren't capable enough to measure it. But that's not true.
The measurement is not the factor stopping us from finding the values. It's because the observation changes the very behavior of the quantum particle.
The Wave-Particle Duality
Earlier the same year, the Schrödinger equation was published. (Read more) This had been the beginning of a new stream of physics, which suggested that the electrons in an atom behaved like a particle and a wave at the same time.
When we examine a particle, we can be sure that it has a few properties like a position and a momentum, but when we think of a wave it has the uncertainty of either the position or momentum.
Only when we look at the full wave we can be certain about the momentum, one single position can't give us this information. This is the property of the wave in general.
But as we understand by the Schrödinger equation, the electron behaves like a wave of probability at first but as soon as we measure it, its probability collapses into one single point suggesting the dual nature of the particle.
Before it's measured, the electron is in the so-called superposition state. This basically means that electrons are at more than one place, in more than one state at a time.
Unless it gets measured.
The Concept of Superposition
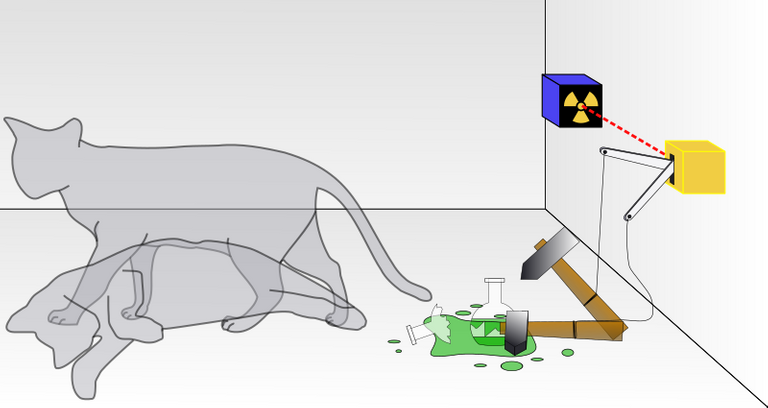
The concept of the superposition can be explained with a Schrodinger's thought experiment involving the famous "Schrödinger's Cat".
Imagine a cat in an isolated box, in which there is a sealed flask of poisoned gas, a radioactive decaying source (single atom), and a mechanism which is triggered by the radioactive decay. It's arranged in a way that if the radioactive source decays, the mechanism is triggered and by the shattering of the poisoned flask, the cat is killed
So here the cat's fate depends upon whether the radioactive source decays or not, which has a 50% chance of decaying.
In this case, the cat is considered to be in a state of superposition (both dead and alive). When we open the box to check (observe), the superposition collapses to one of the possibilities (randomly!).
- The radioactive source has not decayed and the cat is still alive. Yay!!!
- The radioactive source has decayed and the cat is dead. Oh crap!!
 [Image Source]
[Image Source]
The very act of the observation is enough to trigger the collapse.
It's just the measurement, the curiosity of the observer, that killed the cat.
So in the quantum version of the concept, we can say that "the observation collapses the state".
The Mathematical Expression of the Uncertainty Principle
The Heisenberg uncertainty principle is normally written as below:
Where
Δx = Uncertanityinity in position
Δp = Uncertainity inmomentum.
h = Plank Constant
Uncertainty:
E.g. If Δx = 0, then that means we're absolutely sure about the value of x, and if Δx= 1, that would mean the value lies with the margin of 1 unit. Which basically indicated how certain we are of the value of x. (This is to explain the concept, the actual value of x is a standard deviation of the probability in a particular 3-dimensional location)
Thomas Young's Double Slit Experiment
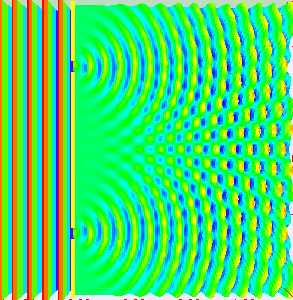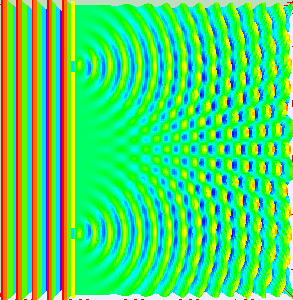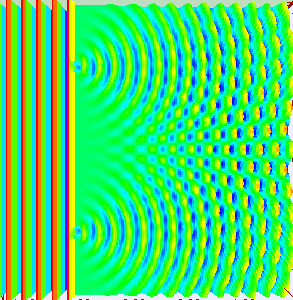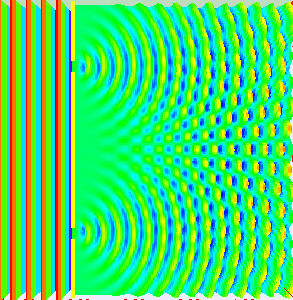
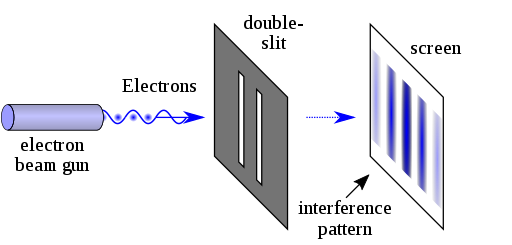
Thomas Young conducted this experiment in 1801 (way before quantum mechanics emerged). The dual nature of the quantum particles can be observed by the double slit experiment, which is conducted by firing electrons at a nontransparent barrier with two very narrow, adjacent slits and observing the screen.
The pattern that emerges on the screen is called interference pattern (A general property of the waves).
The interference pattern is also displayed in the picture on the side
[Image Source]
[Image Source]
The interference pattern suggested that the electrons have wavelike properties.

But if we fire more electrons one by one, the interference pattern emerges again. (Picture on the side.)
[Image Source]
That pattern can only emerge if the single electron is somehow traveling through both of the slits at the same time.
The outcome of the experiment suggests, that every electron is traveling through both of the slits.
But if we decide to observe which slit the electron passes through by adding a detector, the interference pattern does not emerge.
That means when we measure which slit the electron passes through it collapses into a single point particle and passes through a single slit.
As if the electron knows when it's being watched.(Weird. Right?!)
Conclusion
- The quantum object has wave-particle duality. (Properties of both particle and waves)
- A quantum object is in a state called superposition.
- The quantum object's superposition is destroyed by the observation.
- A quantum object reacts differently when observed as if it somehow knows that we're looking at it.(This phenomenon can also be supported by Delayed choice and quantum eraser variations of the double slit experiments)
References: 1, 2, "Final Book on Fundamental Theoretical Physics" by Gunn Quznetsov"
Click here to know more about Suesa's Mentoring Program.Special thanks to @suesa for accepting me as a mentee in her Mentoring program and helping me write this article.

Daily Fun Fact #19 -- "Cats always land on their feet, thanks to physics."
More Information
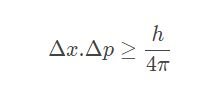
There is an error in your inequality: it is hbar /2 on the right-hand side.
Note also that you cannot apply quantum mechanics to the Schrodinger cats because of the continuous exchanges between the cat and its environment. Maybe this is worthy to specify :)
Except this, your post is good! Maybe would it be good to define exactly what an uncertainty is earlier in the post? What do you think?
wow, thanks a lot for reading. :) I'm glad that you read my post.
I totally miss-put the value of h-bar. it was supposed to be plank constant, instead, I put the reduced planks constant, thank you for noticing :). I will correct it.
It would definitely be better if I define the uncertainty, in a more explanatory way, but I was afraid that people might run away after seeing an equation at the beginning. :P
Again, thank you for your lovely comment. :)
This study calls to mind the idea that quantum particles have a truly random position in three dimensions because their absolute location is fourth dimensional.
Congratulations @arrjey! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
<p dir="auto"><a href="http://steemitboard.com/@arrjey" target="_blank" rel="noreferrer noopener" title="This link will take you away from hive.blog" class="external_link"><img src="https://images.hive.blog/768x0/https://steemitimages.com/70x80/http://steemitboard.com/notifications/payout.png" srcset="https://images.hive.blog/768x0/https://steemitimages.com/70x80/http://steemitboard.com/notifications/payout.png 1x, https://images.hive.blog/1536x0/https://steemitimages.com/70x80/http://steemitboard.com/notifications/payout.png 2x" /> Award for the total payout received <p dir="auto">Click on any badge to view your own Board of Honor on SteemitBoard.<br /> For more information about SteemitBoard, click <a href="https://steemit.com/@steemitboard" target="_blank" rel="noreferrer noopener" title="This link will take you away from hive.blog" class="external_link">here <p dir="auto">If you no longer want to receive notifications, reply to this comment with the word <code>STOP <blockquote> <p dir="auto">By upvoting this notification, you can help all Steemit users. Learn how <a href="https://steemit.com/steemitboard/@steemitboard/http-i-cubeupload-com-7ciqeo-png" target="_blank" rel="noreferrer noopener" title="This link will take you away from hive.blog" class="external_link">here!