
Introduction
Hey guys, I am back with another exciting project. Sorry for the absence, I have been taking some time over the last couple of weeks to read through some whitepapers, I did, however, manage to write an “introduction post” in this time, which you guys should check out if you have not already managed to. I believe we are coming to a stage in the ICO world, where it is becoming harder and harder to find worthwhile projects to get excited about.
But I persisted in my search and found “ClinTex CTi”. This is my opinion is another great use of blockchain. These are the projects which I get excited about, as you know by now. Blockchain is here to revolutionise the world, and what a better use case than the clinical trials industry. So, to give a brief introduction, ClinTex is a Clinical Trials Intelligence (CTi) platform. They will utilise the blockchain to carry out data analytics. They will utilise the Ethereum blockchain to carry out trials in a more efficient and secure manner than traditional methods. For those of you unfamiliar with this space, these trials are extremely data intensive and as we know, blockchain is the solution for large data. ClinTex will also utilise “predictive data analytics” and “machine learning” on their platform, according to Clintex.io, (2018). This article will discuss this project in detail, in order to provide you with all the relevant information on the project. I have taken most of this information from their whitepaper and other sources, I will provide links to these in the further reading section below.
Before we start please, check out this great short overview of the project:
https://www.youtube.com/watch?time_continue=1&v=010VOts8ZLI
Vision
ClinTex, wish to provide an ecosystem for pharmaceutical companies to carry out clinical trials in a more efficient manner, and provide a truly secure and private platform. I for one agree with this vision, why do we need to keep on using outdated tech? when we have the solution to large data on our footstep? What more, is that blockchain has un-parallel security, a prime use within a “data sensitive” industry. This will be a truly secure platform. I believe they will bring all stakeholders into one, easy to use platform, it will aid faster trials combined with better quality processes. ClinTex reckon they can save billions in this industry, by simply making it more efficient.
Pain point
As pointed out in the ClinTex, (2018) whitepaper, pharmaceutical and biotech companies need to carry out trials in order to gain regulatory approval across the world for new drugs & treatments. The largest regulatory authorities comprise of the “Food & Drug Administration (US) and the “European Medicines Agency (EU).
What grasped me within the whitepaper, coming from a perspective of knowing nothing about this process, is how long these clinical trials can take. ClinTex tells us Phase 1 of trials can take up to 7 months and involves safety testing of drugs with volunteers (dosages/effects etc…). Furthermore, phase 2 (efficiency testing) can be as long as 2 years in duration and involves 100s of patients. Phase 2 is where “placebo effect (fake pills)” testing occurs, for those of you who remember your biology in school days; for those of you who are experts, apologies for a basic definition of this. ClinTex tells us that only 1/3 of drugs pass these 2 phases. Next comes global (phase 3) testing and can last several years, this stage is vital to provide a real understanding of the effects of the drugs/treatments. ClinTex tells us 70-90% of drugs pass through this and once passed they can apply for approval for marketing. Lastly, phase 4 testing occurs after consumer sale, which tracks the effects of the said treatment/drug in the long term, with the purpose being data returns.
As one would imagine based on this, the cost of all these stages is astronomical, which is where the pain point lies. ClinTex has designed this ecosystem in order to combat this and provide a more streamlined and efficient process.
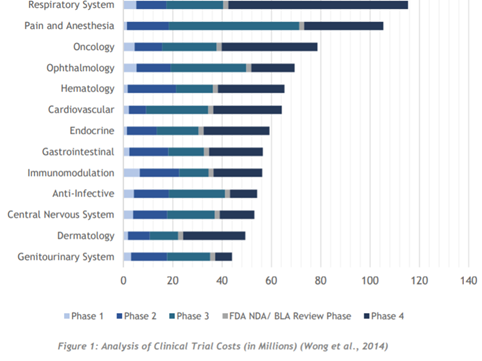
Source: ClinTex, (2018)
One very interesting statistic pulled from the whitepaper states: “the average cost to bring a drug to market is $802 million, however, some estimates lie between $1.3 billion to $1.7 billion” (Collier, 2009). ClinTex, (2018) claim most of these costs relate to data, something which data analytics and machine learning can target.
Solution
From reading this whitepaper, it is clear that ClinTex is targeting these pharmaceutical companies, clinical research organisations and academic institutions who carry out these trials. The ClinTex ecosystem will take all of this data sensitive information and apply various means of predictive analyses, as outlined in their whitepaper. ClinTex claims they have a partnership with “Intellimed”, who are “leaders in the area of data analytics for more than 32 years” (Intellimed, 2018). They plan to drive partnerships with pharma companies by offering free proof of concept via free trials. This will be vital to the success of this platform, as I am sure there will be a lot of inertia to change within the space.
What is more is that there is no competition in this space currently, so ClinTex has first mover advantage in applying blockchain to the clinical trial process. Don’t get confused with various “healthcare/industry projects such as medical chain etc.., these don’t focus on clinical trials. ClinTex quote projects like IBM and Deepmind who are working with blockchain in the medical space, but say these are vastly different from their ecosystem. I am not going to dive into the whole area of competition here, just be ensured, this is a unique first mover project in my opinion. I will note however, it is reassuring to see a project mention a lot of similar projects in their whitepaper and tell us how they differ vastly in their offering.
ClinTex, (2018) quote that if they can even save 1% of costs across the industry, then this would equate to a saving of 3.5 billion dollars. This puts the size of this space into perspective. This 1% saving is easily achievable as if they can make data generation more efficient, this is all that is required. I do however think that they are being extremely conservative in their estimates. ClinTex will combine the efficiency of distributed ledger technology to reduce costs and improve processes, predictive analyses will be used alongside this, so I would suggest this saving will be much higher.
I don’t need to rant on here about the benefits of blockchain, by now we should all know how secure and revolutionary this technology is. As I have mentioned already, ClinTex hopes to be the first to bring this to the Clinical trials industry. This will keep clinical data secure and the immutable nature of the ledger will allow easy attenable results from mandatory audits carried out by regulatory authorities. Blockchain will keep all records for easy management and because of the public nature of blockchain historical data will be easily attainable (via private keys). ClinTex, (2018) also suggest that payments will be made easily and securely via the blockchain and the interoperability nature of blockchain will allow diversification of clinical methods/trials.
This will be a totally secure platform, as is the nature of blockchain, encryption will allow access only to authorised individuals. ClinTex claim from research in their whitepaper, that blockchain could allow years to be knocked off the clinical trials process. I think it is safe to assume that the Ethereum Virtual machine (EVM) is the correct choice to host this platform. The Ethereum network (ERC-20), time and time again has showcased its strength in this space. The utilisation of smart contracts is essential for the ClinTex ecosystem to operate.
A closer look at the Ecosystem
From what I can make out in this whitepaper, this ecosystem has been designed to offset the costly and drawn out process that is the clinical trial process. It will be driven by various machine learning, data analytics and predictive management processes to ensure everything runs efficiently. I also believe they will operate a “pay-per-use” model to drive adoption. All of the various layers of the platform ensure the whole process becomes collaborative and efficient. By having everyone on 1 platform provides many benefits to the process of clinical trials, all data can be easily accessed by important stakeholders. According to ClinTex, (2018) the ecosystem is made up of 7 dApps.
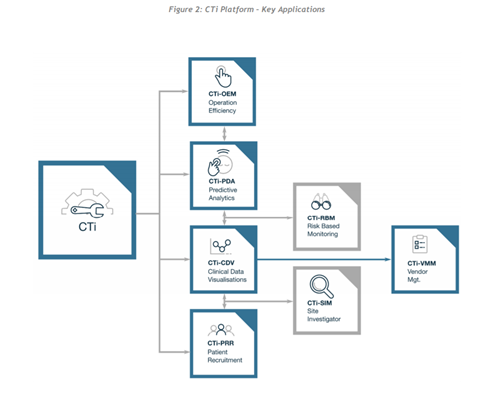
Source: ClinTex, (2018)
CTi-OEM: Operational Efficiency: This will allow for progress tracking of the clinical trial process. Essentially it is the interface, which will also be linked to predictive analytics in order to highlight areas before they delay critical path processes.
CTi-PDA: Predictive Analytics: The whole clinical trial process revolves around trying to get regulatory approval, an efficient process is a key to this. Predictive analyses will allow ClinTex to analyse data and carry out forecasts. Interestingly ClinTex claims that the FDA already approve of similar risk-based approaches but tell us that it is not used widely. This is down to predictive analyses being in its early days of development across all sectors. A great example they give is forecasting a “patient who has a high turnover “during trials.
CTi-CDV: Clinical Data Visualisations (including Statistical Monitoring of Data): ClinTex tells us that traditionally medical reviews of data are done via line listings from the trials. This is a very manual process. This is very time consuming and often causes delays. Blockchain will allow this data to be presented in a way which makes the whole process more efficient (discussed in more detail below).
CTi-PRR: Patient Recruitment & Retention: This describes itself, Clinical trials are a competitive space for “patients”, often is the case that deadlines for testers are missed as it becomes increasingly hard to attract them. The blockchain will allow this process of recruiting and retaining to become effortless, as records can be stored and accessed easily. The platforms analyses tools will allow for the tracking of high-risk patients who may leave, thus allowing ClinTex time to implement retention measures.
CTi-RBM: Risk-Based Monitoring: ClinTex, (2018) claim that the monitoring of accounts costs between 9% & 14% of total trial costs. On top of this, monitors have to manually visit trials to examine the quality of data. ClinTex suggests their risk management process will consider both current and historical data to predict risks. They state they will utilise both “workload management” & “preventing issues” to achieve this. This model will drastically reduce the need for site monitors and provide a less costly process. An example they give is the pain of trying to manage “unevaluable” patients, these are those with a history of not taking trials seriously, blockchain will allow risk mitigation and allow ClinTex to focus risk measures on the most needed areas.
CTi-SIM: Site Investigator: Similar to patient recruitment, there is huge competition for site investigators to carry out trials. ClinTex reimburses these people via their CLX tokens, which is all calculated based on KPIs. Smart contracts will allow milestones (Number of testers, data etc…) to be tracked and transactions automatically carried out. Again, the platform will allow for searching and retention of investigators.
CTi-VMM: Vendor Management: ClinTex tells us that 3rd party vendors are mostly used in trials to look after various aspects of the process. Due to the sensitive nature of this, evaluation is required. This application will monitor data provided by these vendors and will carry out transactions.
In my opinion, ClinTex offers a platform which will align itself with the needs of this industry. They state in their whitepaper that their “Clinical Trials intelligence Platform” will focus on end-end management for users. All of the functions I described above will essentially by managed via dashboards, to ensure user-friendliness of the platform.
As somebody who works in an industry which relies heavily on progress reporting on projects, I can ensure you all that nobody I have ever worked for wants to read through 100s of pages of excel data sheets. Most companies want a simple and easy to read report comprising of charts. I would assume this industry follows the same requirement. From what I can see in the whitepaper, ClinTex will offer dashboard reporting across every facet of the platform. See below examples of the dashboards for the “Vendor application” and “risk monitoring application” of the platform.
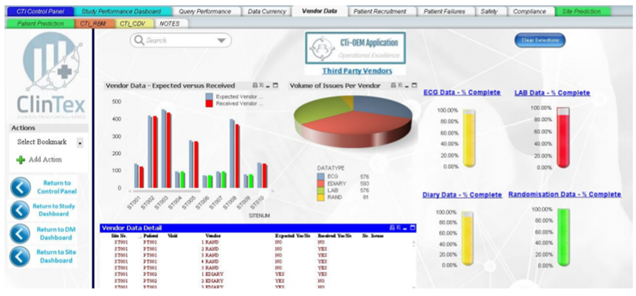
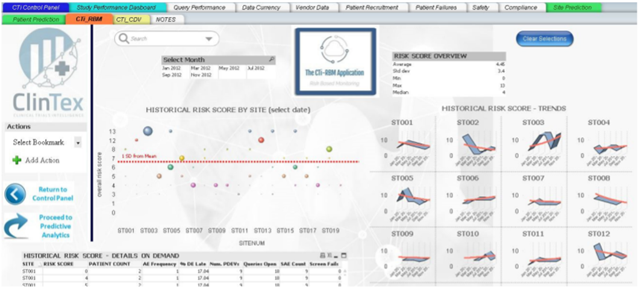
Source: ClinTex, (2018)
I have to admit; these dashboards seem like they are put together very well. You can check them out in the whitepaper yourself. I am not going to discuss them in any detail here. ClinTex provides some very detailed explanations of how this platform works in their whitepaper.
I would like to know more of how these reports are pulled together. In my own daily life, the data that is compiled into these dashboards is drawn from hundreds of excel pages. I feel that this approach will position ClinTex well in the market. I am particularly impressed by the risk-based monitoring applications, as this will allow pharma companies to adopt a more “risk-based” approach to clinical trials, as outlined by ClinTex, (2018). Large data is the lifeblood of this industry and ClinTex have shown they have thought about they will handle this.
Overall this platform is very exciting, smart contracts will be central to how it operates. ClinTex, (2018) also tell us that will utilise “chain-link” for payments to investigators and vendors based on progress. Chain link is the worlds first decentralised oracle network which allows smart contracts to utilise data feeds for payments. Also, ClinTex will use “storj” as its decentralised storage provider. From what I know of this, this is a fully secure data storage facility which fully encrypts data, this means private keys are needed to access it.
Token Economics

Source: ClinTex, (2018)
The CLX token, as described in the ClinTex, (2018) whitepaper will have a lot of uses on the platform. If you examine the roadmap below, the token will have a use case on each roll out/phase of the delivery of the project. The token is a utility token for use on the platform so they will be used to carry out transactions on each application of the platform.
Furthermore, ClinTex tells us that access rights will be set up and dictated by token balances (amount yet to be announced). I believe the access rights will be a form of staking within their integrated wallet, I see in their whitepaper that tokens will be subject to “12 months” staking terms. I believe this in effect will provide a lot of price stabilisation.
ClinTex, (2018) furthermore state that they will aim to attract major pharmaceutical partners by retaining 3% of tokens. This will be essential for the proof of concept of the CTI model. I also believe that tokens will be subject to a reducing supply as more and more tokens will need to be staked as the rollout continues. Licensing periods will also dictate these staking amounts/periods, thus ensuring a stable token price I reckon.
What I find very interesting is that to drive adoption of this platform, ClinTex will set up a brokerage service (ClinTex Uk) to take in fiat payments from large pharma companies, they will, in turn, convert these payments to the CLX token. This will be a fee-based service. Also, very interestingly, ClinTex envisage most companies renewing licenses to ensure tokens are constantly locked up (rarity) if they cancel after the year 25% of these tokens will be burned (destroyed) before the rest are returned to the circulation supply (market). This is a great model and should ensure price stabilisation.
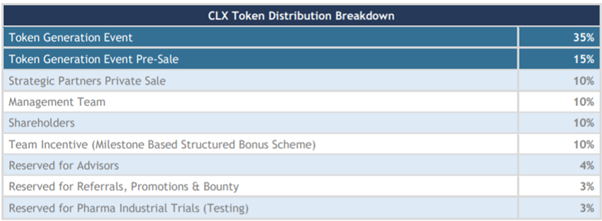
Source: ClinTex, (2018)
The use of funds is outlined in the chart below. This is a very healthy distribution of ICO funds to ensure the success of the project development.
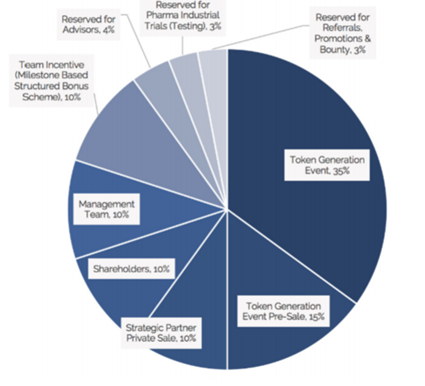
Source: ClinTex, (2018)
Use of funds is as followed:
- 35% platform development: This is perhaps the most essential use of funds and I am happy to see this amount allocated for this.
- 30% clinical and analytical development: This is another vital element to to ensure the project is a success.
- 2% research grants: This is great as it is needed to drive adoption.
- 15% marketing: This will be vital to ensure the project has the reach it requires.
- 10% legal and accounting: This will also be vital to ensure they meet the demands of regulators.
- 8% operational: The nuts and bolts of the organisation.
Roadmap
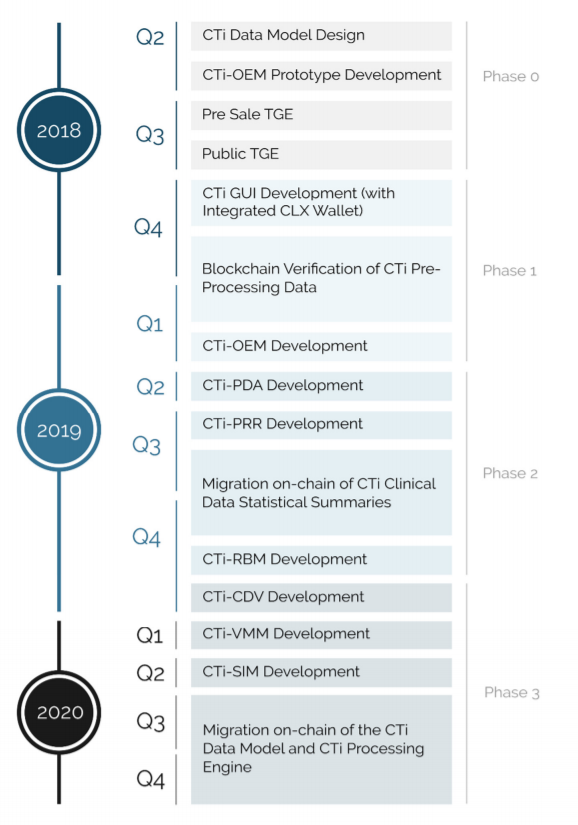
Source: ClinTex, (2018)
I have to admit, that this is a very impressive roadmap. You can see this team have a very well-spaced out timeline, in order to deliver this platform. As can be seen, most of the development work on the platform will take place during 2019 and into early 2020. I believe this is a very ambitious project, one which is being tailor-made to cater to a huge industry. What is more, the first user advantage gives ClinTex a huge competitive advantage. I think this roadmap will provide investors will greatly value on their investment.
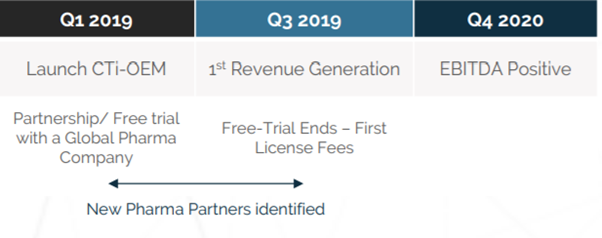
Source: ClinTex, (2018)
ClinTex will roll this project out in 3 phases:
- Phase 1: Within this phase, clinical data will be able to be stored in the cloud and processed via the blockchain. This will also be the period in which the CLX token is introduced. Users will be required to use CLX tokens staked in wallets as a term of their license.
- Phase 2: According to ClinTex, (2018) this is where hash tables will be introduced. This will provide a more immutable platform. Further improvement to storage will also be introduced here.
- Phase 3: This is when ClinTex envisage the platform will transition to a fully decentralised solution. They also mention the introduction of another native token here which will conform to the ERC721 standard. They state this will be needed for data input into the platform. I do not know any more about this but I believe it is needed for the smart contract mechanics. Overall Phase 3 will deliver the full ecosystem.
The Team
The team I feel can deliver this project. The 19-person team have a large scope of experience across the pharmaceutical & clinical trial industry. What I love about the makeup of this team is that they are experts in the industry and have combined this experience with expertise in blockchain, software, and analytics. I am not going to talk much about the team here because I feel if a project has a good enough vision then a team can deliver it. I will say however that I am impressed with what I read in this whitepaper, regarding each individuals experience.
Conclusion
As I mentioned in my introduction it is becoming increasingly difficult to find good ICO’s. I am a strong believer that this market is simply too immature and untested to accommodate most of the ICOs we see occurring today, and this has nothing to do with the team or somebody’s experience, it is down to the fundamentals of the market. Saying this, we still have these important projects striving ahead with roll outs of their projects, projects with a real use case. When I say use case I am referring to the ability of blockchain to better society, I can think of no better use case of blockchain than to aid in the development of medicines and bring efficiency to the clinical trials process. It is these projects, which are pushing boundaries, where I am putting my money, these are going to be the big winners in the future.
ClinTex, I feel have a very impressive and ambitious project here. The stats they put forward in their whitepaper are very convincing. They will combine the efficiency and safety of blockchain with predictive analyses and machine learning and revolutionise this industry. Their ecosystem, which is planned to roll out throughout 2019 and into 2020 is perhaps one of the best laid out platforms I have seen in a long time. The team here are striving to bring real change to the world, which in my view is what blockchain is all about. What is more, is that ClinTex has first user advantage in the clinic trials industry. The CTi platform being developed, in my opinion, will achieve a more collaborative process by bringing all stakeholders together in one ecosystem. I strongly believe this revolutionary project will bring huge savings and untold efficiency to this space.
Final word
Lastly guys, I just want to stipulate to you that you should not take any of this as investment advice. I am not a financial advisor and urge you all to do your own research when considering any investment, this space can be very unforgiving and is very speculative in nature. Never follow somebody blindly; my job is to showcase projects I see with real potential that is all. I hope you enjoyed this article and I will aim to find the next amazing project in due course. I thank you all for your support and ask for you to share my work. I would be more than happy to discuss any questions you may have in the comments section.
Further reading
- ClinTex Website: https://www.clintex.io/
- ClinTex Whitepaper: https://www.clintex.io/Clintex_CTi_Whitepaper.pdf
- ClinTex Lite paper: http://clintex.io/Clintex_CTi_Litepaper.pdf
- ClinTex One pager: https://clintex.io/Clintex_CTi_OnePager.pdf
- ClinTex Technical Paper: https://clintex.io/Clintex_CTi_Techpaper.pdf
- ClinTex Telegram: https://t.me/ClinTexCTi
- ClinTex Twitter: https://twitter.com/ClinTexCTi
- ClinTex Reddit: https://www.reddit.com/r/ClinTexCTi/
- ClinTex LinkedIn: https://www.linkedin.com/company/eclintex-ltd/
- ClinTex Medium: https://medium.com/clintexcti
References
- Clintex.io. (2018). [online] Available at: https://www.clintex.io/Clintex_CTi_Whitepaper.pdf [Accessed 21 Aug. 2018].
- Collier, R. (2009) ‘Rapidly rising clinical trial costs worry researchers.’, CMAJ: Canadian Medical Association journal = journal de association medical canadienne. Canadian Medical Association, 180(3), pp. 277–8. doi: 10.1503/cmaj.082041.
- Intellimed. (2018). Home. [online] Available at: https://www.intellimed.com/ [Accessed 22 Aug. 2018].
You just took ico project reviews to a new level. Great work here @cryptoguru1. Keep it up
Thank you for your kind words. Aim to aid people as best I can
Excellent and well articulated summary of the CTi project - thank you
No problem, Great to find such a good project. Wish you all the best with the sale
Fantastic and well articulated article. Nice idea on the blockchain. Thumbs up
Thanks Karl, your support on my work is much appreciated
Wow! This is a breathtaking and a well articulated summary of the Clintex project. You deserve all the accolades you'll get on this work. Thumbs up
Thank you Fenderr, much appreciated for this comment. hopefully others see it that way and do their own reading about it. Not often such a good project comes around
Congratulations @cryptoguru1! You have completed the following achievement on Steemit and have been rewarded with new badge(s) :
<p dir="auto"><a href="http://steemitboard.com/@cryptoguru1" target="_blank" rel="noreferrer noopener" title="This link will take you away from hive.blog" class="external_link"><img src="https://images.hive.blog/768x0/https://steemitimages.com/70x80/http://steemitboard.com/notifications/votes.png" srcset="https://images.hive.blog/768x0/https://steemitimages.com/70x80/http://steemitboard.com/notifications/votes.png 1x, https://images.hive.blog/1536x0/https://steemitimages.com/70x80/http://steemitboard.com/notifications/votes.png 2x" /> Award for the number of upvotes <p dir="auto"><sub><em>Click on the badge to view your Board of Honor.<br /> <sub><em>If you no longer want to receive notifications, reply to this comment with the word <code>STOP <p dir="auto"><strong><span>Do not miss the last post from <a href="/@steemitboard">@steemitboard:<br /> <a href="https://steemit.com/veterans/@steemitboard/steemitboard-and-the-veterans-on-steemit-the-first-community-badge" target="_blank" rel="noreferrer noopener" title="This link will take you away from hive.blog" class="external_link">SteemitBoard and the Veterans on Steemit - The First Community Badge. <blockquote> <p dir="auto">Do you like <a href="https://steemit.com/@steemitboard" target="_blank" rel="noreferrer noopener" title="This link will take you away from hive.blog" class="external_link">SteemitBoard's project? Then <strong><a href="https://v2.steemconnect.com/sign/account-witness-vote?witness=steemitboard&approve=1" target="_blank" rel="noreferrer noopener" title="This link will take you away from hive.blog" class="external_link">Vote for its witness and <strong>get one more award!Great