
Image Source
Dry cell, we also know this as AA cell. This post is based on the structure and function of the dry cell. A Dry cell is a refined form of Leclanché Cell. In the Leclanché Cell, the fluid is filled, which makes it difficult to use. The dry cell has been built to overcome this difficulty so that no type of fluid is used. It was developed in 1888 by the German scientist Carl Gassner[1] [2]. Dry cells are in the form of electrolytes of the paste. In which there is enough moisture to allow electric current only.
In the dry cell, Zinc has a cylindrical jar that acts like a Negative Pole. Within this, a paste made from a mixture of Ammonia Chloride, zinc chloride and gum is filled. In the middle of the jar, there is a bag of muslin that contains a mixture of Manganese dioxide and carbon powders. A carbon rod is mounted in between this, with a brass cap on its upper end. This rod of carbon acts as a Positive Pole. The open part of the jar is sealed. There are subtle holes on it so that the ammonia gas can get out. The curved bottom of the cell is wrapped in paper and it is made insulator. The base remains open which acts as the Negative pole for the deaf circuit.
When the carbon and zinc rod is added to the circuit then in contact with electrolytes, zinc atoms are ionized by giving two electrons.
Zn → Zn2+ + 2e-
Ammonium chloride (NH4Cl) in the electrolyte is thus ionized:
2NH4Cl → 2NH4+ + 2Cl-
Zinc ions make zinc chloride combined with chlorine ions.
Zn2+ + 2Cl- → ZnCl3
Ammonium ions (NH4+) flow towards the carbon rod and become Indifferent by taking an electron from the carbon rod, ammonium is converted into hydrogen gas.
2NH4+ + 2e- → 2NH3 + H2
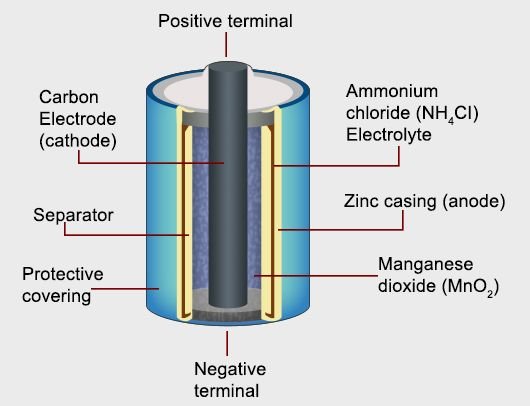
Image Source
Manganese dioxide converts hydrogen gas into water, which does not cause the action of polarization.
2MnO2 + H2 → Mn2O3 + H2O
Cell Reaction:[3]
Zn + 2NH4Cl + 2MnO2 → ZnCl2 + 2NH3 + Mn2O3 + H2O + Energy
The procedure of dry cell is similar to the working method of Leclanché Cell. The Electromotive force of the cell is 1.5 volt. It gives a stream of 0.25 Ampere.
References:
- 1888 by the German scientist Carl Gassner
- https://web.archive.org/web/20080210024627/http://chem.ch.huji.ac.il/history/gassner.html
- Cell Reaction
Followed @crackie